Complying with the FDA’s Good Manufacturing Practices regulations, as established in Quality Plans, has become increasingly complex every time. MadgeTech has simplified this process by including IQ/OQ/PQ protocols with its MadgeTech 4 Secure software package.
This feature saves time and eliminates the need to develop internal software validation procedures. The MadgeTech IQ/OQ/PQ protocol complies with FDA guidelines and good manufacturing practices. Additionally, MadgeTech Secure offers a validation workbook to help the user verify the software’s functionality.
Installation Qualification (IQ)
- Description of the MadgeTech system.
- Verification that all equipment, accessories, and software received from MadgeTech are in good condition.
- Verification that the documentation is complete.
- Verification that the MadgeTech equipment is properly installed.
- Verification that the MadgeTech 4 Secure software is installed correctly at the workstations.
- Verification of basic communication between MadgeTech data recorders and the destination workstations.

Operational Qualification (OQ)
- Functional verification of MadgeTech data recorders.
- Information on handling and maintenance by the MadgeTech team.
- Operational procedures for the MadgeTech team.
- Verification of communication between data recorders and MadgeTech workstations.
- Verification of hardware functionality of the data recorder.

Performance Qualification (PQ) Recommendations
- Additional handling precautions to maintain the precision of the MadgeTech equipment.
- Preventive maintenance information provided by the MadgeTech team.
- Verification of calibration at the site.
- Comparison of values reported with a known standard.
- Verification of acceptable performance in the destination system.

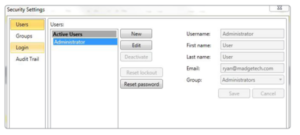
Administrator and User Configuration
Users can be assigned roles or levels of access, such as administrator or user. Administrators have access to all security settings, while users only have access to communicate with data recorders and analyze data.
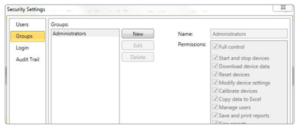
Groups
Users and administrators can be assigned to groups and easily managed using various permissions.
Electronic Signature
By clicking the Electronic Signature button, users and administrators can add electronic signatures. The electronic signature contains the signer’s printed name, the date and time of signing, and the meaning of the signature.
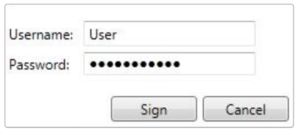
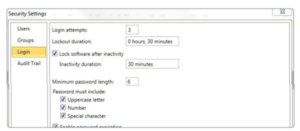
Login
Login attempts and lockout duration can be assigned in the login tab. There are numerous password and account settings that the administrator can configure, such as password complexity and user account status. The user administration tab is only available for administrative users.
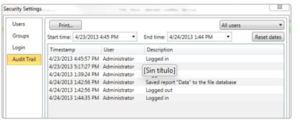
Audit Log
An automatic audit log is maintained with information such as who logged in or out, what files were downloaded, saved, deleted, printed, etc. Each log entry includes the date and time and user information.
If you would like more information about these protocols or MadgeTech Secure Software, or if the data loggers compatible with this software, do not hesitate to write to our Technical Support email: soporte@logicbus.com
sales@logicbus.com | support@logicbus.com | +1 619 616 7350 | Start conversation